The First Principal Energy Level Is Called the
Which principle energy level contains the f sublevel. All p orbitals are shaped like.

How Many Sublevels Are In The Second Energy Level At Level
The 3s orbital differs from the 2s orbital in that it is.
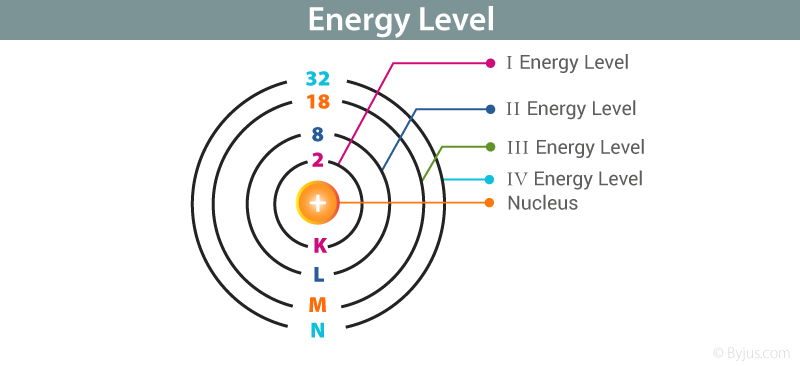
. The first four energy levels are shown here. A quantum mechanical system or particle that is boundthat is confined spatiallycan only take on certain discrete values of energy called energy levels. The p - Sublevel can hold a maximum of.
On the periodic table elements that behave in a similar manner are found in the same. Experts are tested by Chegg as specialists in their subject area. An electron is excited from the n1 ground state to the n3 state in a hydrogen atom.
Electrons that are in the highest energy level for the atom are called the _____ valence electrons. Who are the experts. GENERAL CHEMISTRY 1 LAB EXAM 5 The first principle energy level is called the.
View Quiz4docx from CHM MISC at University of Florida. Select oneaPrinciple one state bGround state cAtomic state dHunds state Feedback The correct answer isGround state 2. The s - Sublevel can hold a maximum of.
This contrasts with classical particles which can have any amount of energy. The first principal energy level of the hydrogen atom contains only an. The term is commonly used for the energy levels of the electrons in atoms ions or molecules which are bound by the electric field of the nucleus but.
How many sublevels does Level 1 contain. E n R h 1 n 2. An element with 8 electrons in its outermost main energy level is called a.
However because of the pauli exclusion principle the other electron must have an opposite spin. Each vertical column on the periodic table is called a. We review their content and use your feedback to.
How many orbitals does an f sublevel contain. The second electron will go in the same obital as the first because in the principle energy level 1 there is only one sublevel an s sublevel. The correct sequence in ascending energies of atomic sublevels is.
Principal energy levels in their ground state. The energy of an electron when it is far away from the influence of the nucleus is taken as zero. Each Principle Energy Level is comprised.
A model of an atom shows eight electrons in rings that represent different energy levels. Where R H is called Rydberg constant whose value is 21810 18 J. Sylviaruthwilson1971 sylviaruthwilson1971 1 week ago Chemistry College answered What is the first principle energy level called 2 See answers Advertisement Advertisement freshman14 freshman14 Answer is s orbital.
The first energy level is also called level K. Of different Sublevels which are. The first principle energy level is called the.
Principal quantum number of an electron existing in such a stationary state is taken as n. The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy. What is the first principle energy level called Get the answers you need now.
S p d f. What is the maximum number of electrons that can occupy the first principal energy level. Electrons in the outermost energy level are also called Valence electrons.
The second level is called level L third energy level as M and so on. The d - Sublevel can hold a maximum of. How many electrons are in each energy level1 point 1two in the first energy level six in the second energy level 2eight in the first.
Which principle energy level is closest to the nucleus and has the lowest energy. Question text The first principle energy level is called the. Which sublevels does Level 2 contain.

How Many Sublevels Are In The Third Energy Level At Level
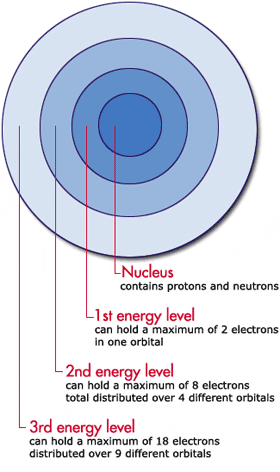
Interactives The Periodic Table It S Elementary For A Mad Scientist

Energy Level Principal Quantum Number Bohr S Atomic Model Physics
No comments for "The First Principal Energy Level Is Called the"
Post a Comment